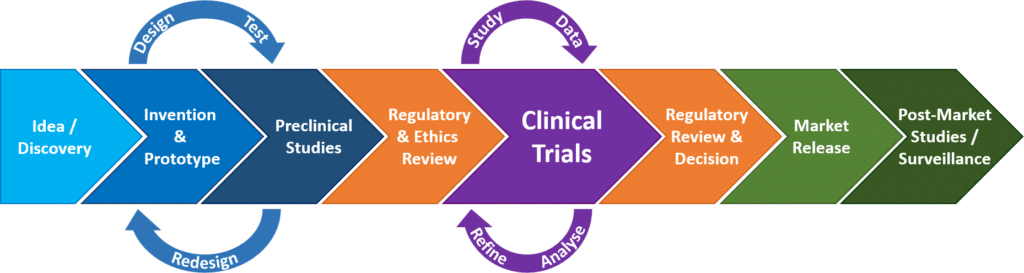
In our previous article introduction to clinical trials we talked about how clinical research is broken up into a series of phases each with a different distinct purpose beginning with though not always conducted pilot studies then safety testing then efficacy testing then.
Medical device clinical trials ppt.
In 2008 roughly 42 percent of the world s total.
Clinical trials of medical devices dr dwaipayan sarathi chakraborty pgt dept.
Medical device clinical trials phases vs stages.
Identify the 10 sponsor responsibilities discussed in the presentation.
What we should know medical device clinical trials.
Overview introduction definition classification global and indian market salient features of development phases of medical device clinical trial types of investigational device studies.
Contrast the role of monitoring with the.
Before doing a clinical trial investigators conduct preclinical research using human cell cultures or animal models.
Clinical trials of medical devices 1.
Of pharmacology ipgme r kolkata 2.
Iso 14971 4 describes the product risk management process fig.
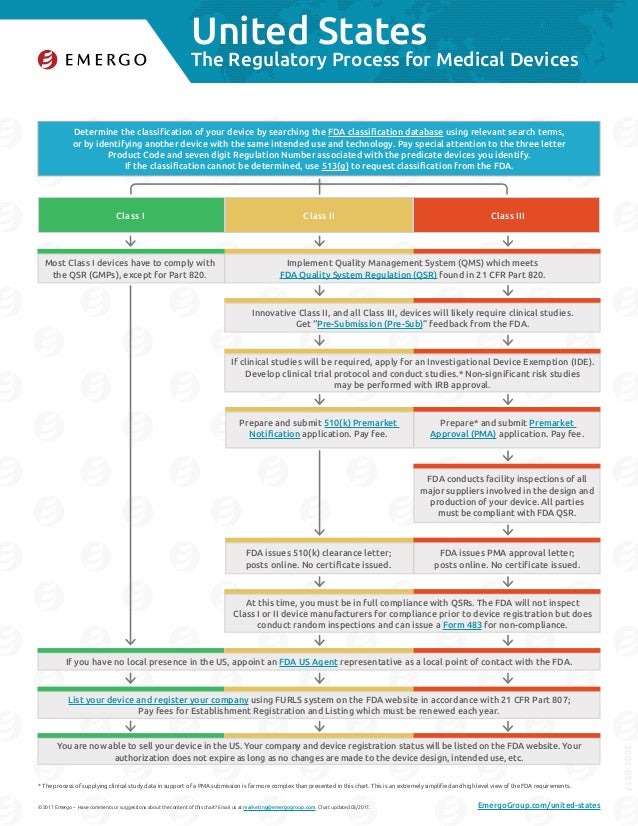
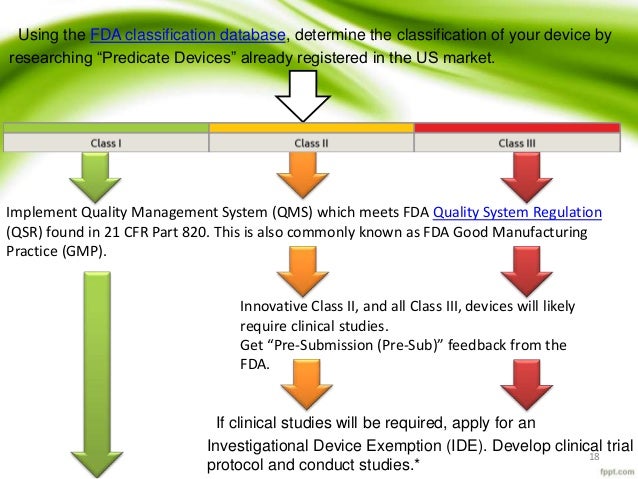
Fda and the ide process owen faris ph d.
1 clinical trials for medical devices are predominantly conducted to identify the safety and performance of.
Incidents in which a device may have caused or contributed to a death or serious injury must to be reported to fda under the medical device reporting.
In the context of medical devices a clinical trial or a clinical investigation can be defined as any systematic investigation or study on one or more human subjects undertaken to assess the safety or performance of a medical device.
Define medical device research sponsor z.
The tips in this article can help the clinical trial manager better execute project risk management for their clinical trials with the aim of providing clinical data meeting pre and postmarket requirements.
Medical device reporting 21 cfr part 803.
The clinical trial manager and medical device product risk management.
In medical device research define good clinical practice gcp outline the goals of gcp provide a historical perspective on gcp outline fda regulations relating to gcp.
What we should know abstract the medical device industry comprises a major sector of the overall healthcare industry representing a more than 100 billion industry in the u s.
For example they might test whether a new medication is.